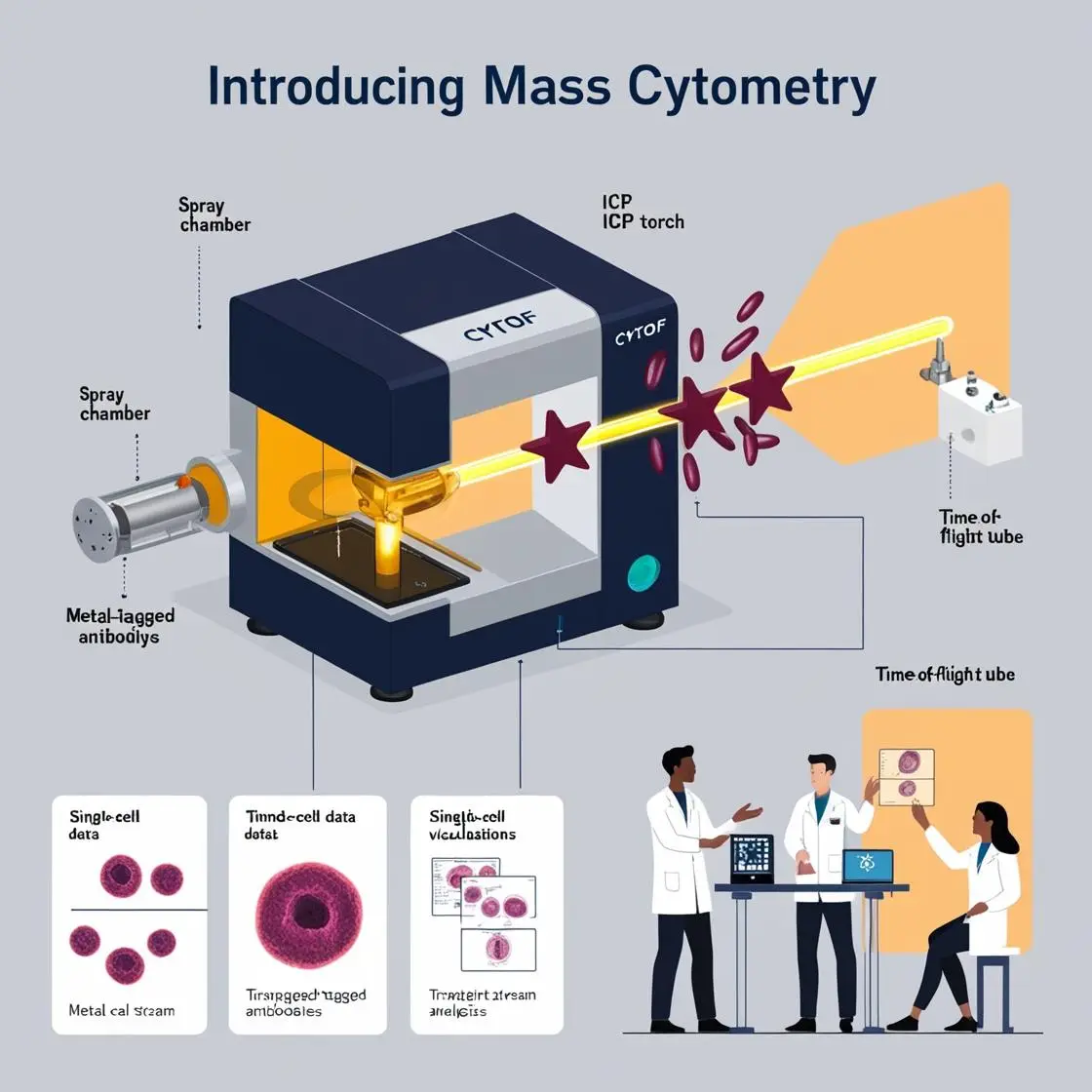
In mass cytometry, sample preparation is an art form that can make or break an experiment. Let’s dive into the intricate techniques transforming our cellular specimens into data-rich marvels.
Cell Fixation and Permeabilization Methods
A cell’s journey through a mass cytometer begins long before it reaches the instrument. Fixation and permeabilization are crucial steps that preserve cellular architecture and allow access to intracellular antigens.
Fixation: This step “freezes” cells in time, preserving their structure and protein localization. Common fixatives include paraformaldehyde (PFA) and methanol.
- PFA is excellent for maintaining cell morphology but can mask some epitopes.
- Methanol, while harsher, can expose specific nuclear antigens more effectively.
Permeabilization: This process creates tiny holes in the cell membrane, allowing antibodies to access intracellular targets.
Behbehani et al. (2014), from Nolan group, introduced a clever saponin technique for transient partial permeabilization. This method allows for cellular barcoding before surface marker staining, a significant advancement in sample multiplexing.
Pedersen et al.’s 2022 study in Cytometry Part A, “Mass Cytometry Assessment of Cell Phenotypes and Signaling States in Human Whole Blood,” represents a significant contribution to mass cytometry methodology. The authors conducted a comprehensive evaluation of sample preparation techniques for whole blood analysis using mass cytometry. They compared various fixation and permeabilization protocols, assessing their impact on both surface and intracellular marker detection. Their findings revealed that a combination of formaldehyde fixation followed by methanol permeabilization provided optimal results for simultaneous analysis of surface markers and intracellular phospho-proteins. This work offers valuable insights for researchers aiming to optimize their mass cytometry protocols, particularly for studies involving both cellular phenotyping and signaling state analysis in whole blood samples.
Advantages of Permeabilization in Mass Cytometry:
- Access to intracellular targets
- Improved signal-to-noise ratio for some markers
- Enables simultaneous analysis of surface and intracellular proteins
- Significantly extends sample storage time compared to flow cytometry
Longevity of Permeabilized Samples: One key advantage of mass cytometry over flow cytometry is the extended storage time of fixed and permeabilized samples. While flow cytometry samples typically need to be analyzed within 24-48 hours, adequately prepared mass cytometry samples can be stored much longer.
More impressively, a study from Standard BioTools shows a stability of the samples for 120 days.

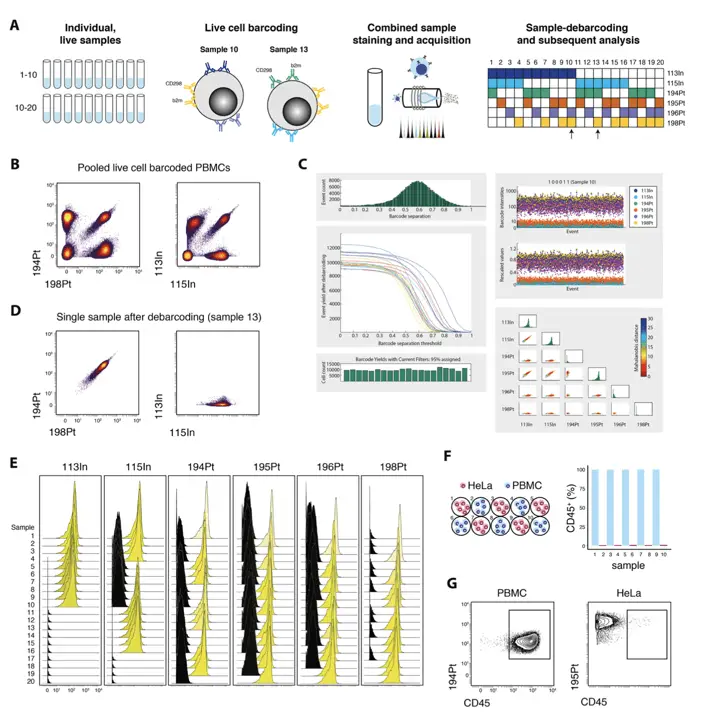
Barcoding Strategies for Multiplexing
Barcoding is a powerful technique that allows multiple samples to be combined and analyzed simultaneously, reducing inter-sample variation and acquisition time.
The landmark paper by Zunder et al. (2015) in “Nature Protocols” introduced a palladium-based barcoding method that has become a staple in many mass cytometry workflows. This technique uses unique combinations of palladium isotopes to label different samples, allowing for up to 20 samples to be combined.
Advantages of Barcoding:
- Reduced inter-sample variation
- Increased throughput
- Conservation of rare or expensive reagents
Drawbacks of Barcoding:
- Potential loss of rare cell populations due to sample pooling
- Increased complexity in data analysis
- Some barcoding reagents may interfere with specific cellular epitopes
A real-world example comes from a study by Hartmann et al. (2018) published in “Scientific Reports.” In a mouse model, they used a 10-plex barcoding strategy to analyze immune cells from multiple tissues. This approach allowed them to directly compare immune responses across different organs while minimizing technical variation.
Importance of Proper Sample Handling
The journey from living tissue to analyzed data is fraught with potential pitfalls. Proper sample handling is crucial for obtaining reliable results.
Regular quality checks are essential. Including control samples and using standardization beads, as described by Tricot et al. (2015) in “Cytometry Part A,” can help ensure consistent performance across experiments.
A cautionary tale comes from myself who once left a precious patient sample at room temperature overnight. The resulting data showed massive activation of stress pathways, nearly derailing a critical experiment. This experience underscores the importance of meticulous sample handling protocols.
In the intricate dance of mass cytometry, sample preparation sets the stage. From the gentle art of fixation and permeabilization to the strategic brilliance of barcoding, each step plays a crucial role in unlocking the cellular secrets within our samples. The ability to store samples for extended periods – up to 100 days in some cases – offers unprecedented flexibility in experimental design and data acquisition, a clear advantage over traditional flow cytometry. As we refine these techniques, we edge ever closer to a complete understanding of the cellular orchestra that composes life.
In the lab, keeping track of samples is like juggling eggs – one slip, and you've got a mess on your hands. After a day of prepping 200 samples, I found myself one short. Queue the lab-wide manhunt, turning drawers inside out like a magician searching for a misplaced rabbit. The missing tube? Nestled in the centrifuge, discovered the next morning. Relieved but reckless, I stained it solo, ignoring its overnight room temperature siesta. Rookie mistake. The resulting data looked like abstract art – interesting, but scientifically useless. Lesson learned: in mass cytometry, proper sample handling isn't just important – it's the linchpin of reliable results. Treat your samples like rare orchids: keep them cool, handle with care, and never, ever leave them spinning alone overnight.
Guillaume Beyrend