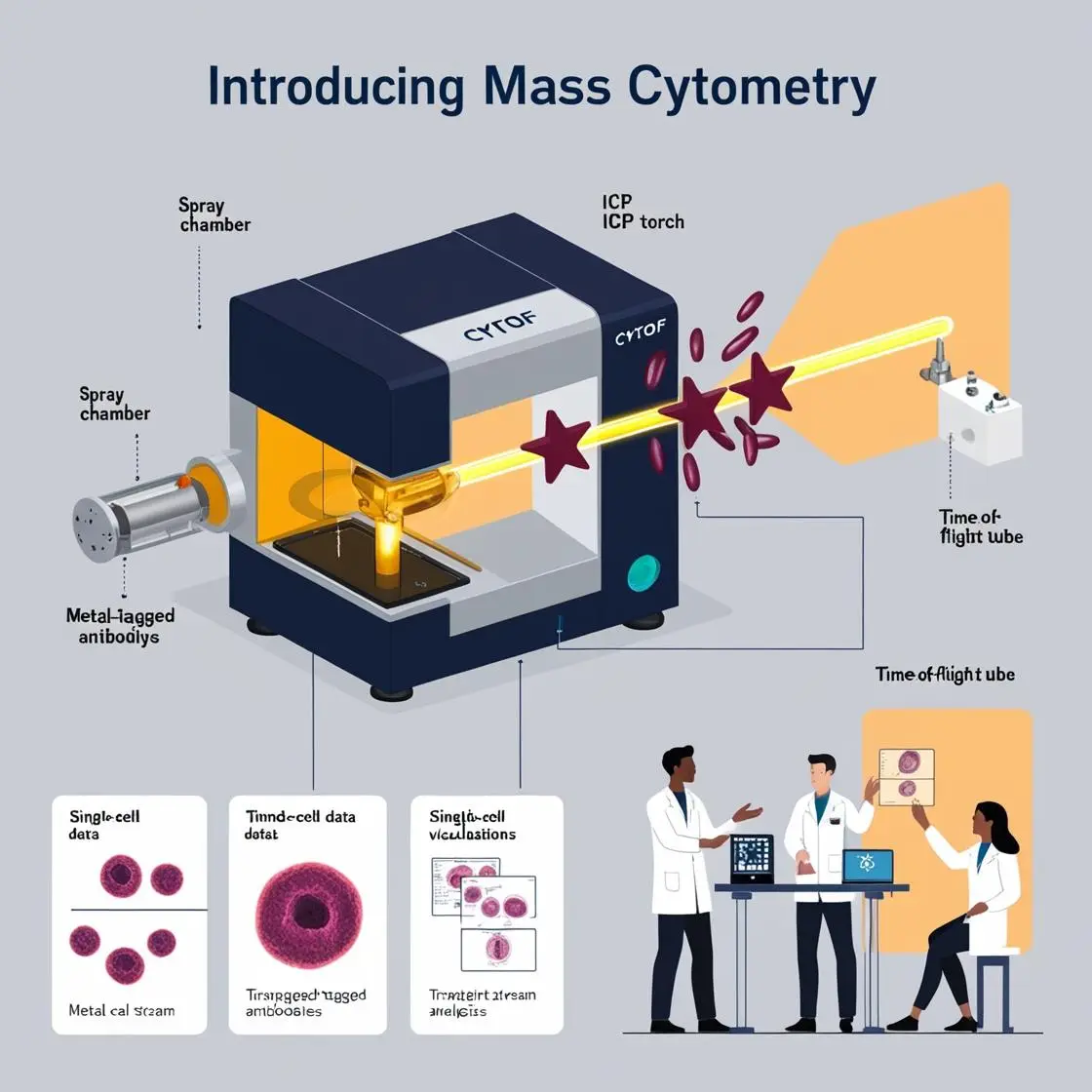
In mass cytometry, metal-conjugated antibodies are the secret agents of cellular interrogation. These clever molecular constructs allow us to peer into the inner workings of cells with unprecedented detail. Let’s dive into the fascinating world of metal labeling and antibody conjugation.
Chemistry of Metal Labeling
The magic of mass cytometry begins with the marriage of metals and antibodies. This union is not a simple affair but rather a carefully orchestrated chemical dance.
Lou et al.’s pioneering work (2008) laid the groundwork for polymer-based elemental tagging in their paper “Polymer-Based Elemental Tags for Sensitive Bioassays”. They introduced a method using metal-chelating polymers to attach multiple metal ions to a single antibody. This approach significantly amplified the signal, making detection more sensitive.
Fast-forward to more recent times, and we see refinements in this chemistry. For instance, Han et al. (2020), in their paper “Metal-Labeled Monoclonal Antibody Conjugates for Use in Mass Cytometry” (Nature Protocol), introduced improved metal chelators. The authors meticulously detail protocols for conjugating rare earth metals to antibodies, enabling the creation of high-dimensional panels with up to 50 parameters for single-cell analysis. They provide three optimized protocols for conjugating monoclonal IgG antibodies with 48 high-purity heavy-metal isotopes: (i) 38 isotopes of lanthanides, 2 isotopes of indium, and 1 isotope of yttrium; (ii) 6 isotopes of palladium; and (iii) 1 isotope of bismuth. I used the protocol for Bismuth conjugation in my own papers and it works very well.
Restricted content
You must be logged in and have a valid subscription to see this content. Please visit our subscription page for more info. If you are already a VIP member, be sure you are logged in with the same email address you made your purchase.
Picture this: there I was, cradling a tiny vial worth more than my entire grad school wardrobe. No, it wasn't liquid gold or a rare perfume – it was 3 mL of metal-conjugated antibodies, a cocktail so precious it made my hands shake like I'd had a few too many espressos. You see, in the world of mass cytometry, fresh is best. So, I decided to go big or go home – 200 samples big, to be exact. It was like preparing for a gourmet dinner party, but instead of farm-fresh vegetables, I was dealing with antibodies that cost more per milliliter than vintage champagne. The day of staining was like a scientific flash mob. Suddenly, the lab was swarming with 10 eager helpers, pipettes at the ready. It was a beautiful sight – a ballet of lab coats and precision timing. Who says scientists can't coordinate? Why all this fuss, you ask? Well, acquiring everything in one heroic week-long marathon meant the CyTOF's detector would have the same sensitivity throughout. It's like making sure the judge in a singing competition doesn't get a hearing aid halfway through – consistency is key! Sure, we could have spread it out over a year, but where's the fun in that? Plus, with the rate at which lab equipment gets upgraded, we might as well have been comparing apples to very expensive, highly sophisticated oranges. So there we were, a team of scientific commandos, armed with pipettes and a small fortune in antibodies, ready to stain our way into the annals of mass cytometry history. It was exhausting, exhilarating, and probably a bit mad – but hey, that's science for you. Just remember, the next time you see a beautifully consistent dataset, spare a thought for the bleary-eyed researcher who might have just pulled off the equivalent of a cytometric Olympics. Medal ceremony, anyone?
Guillaume Beyrend