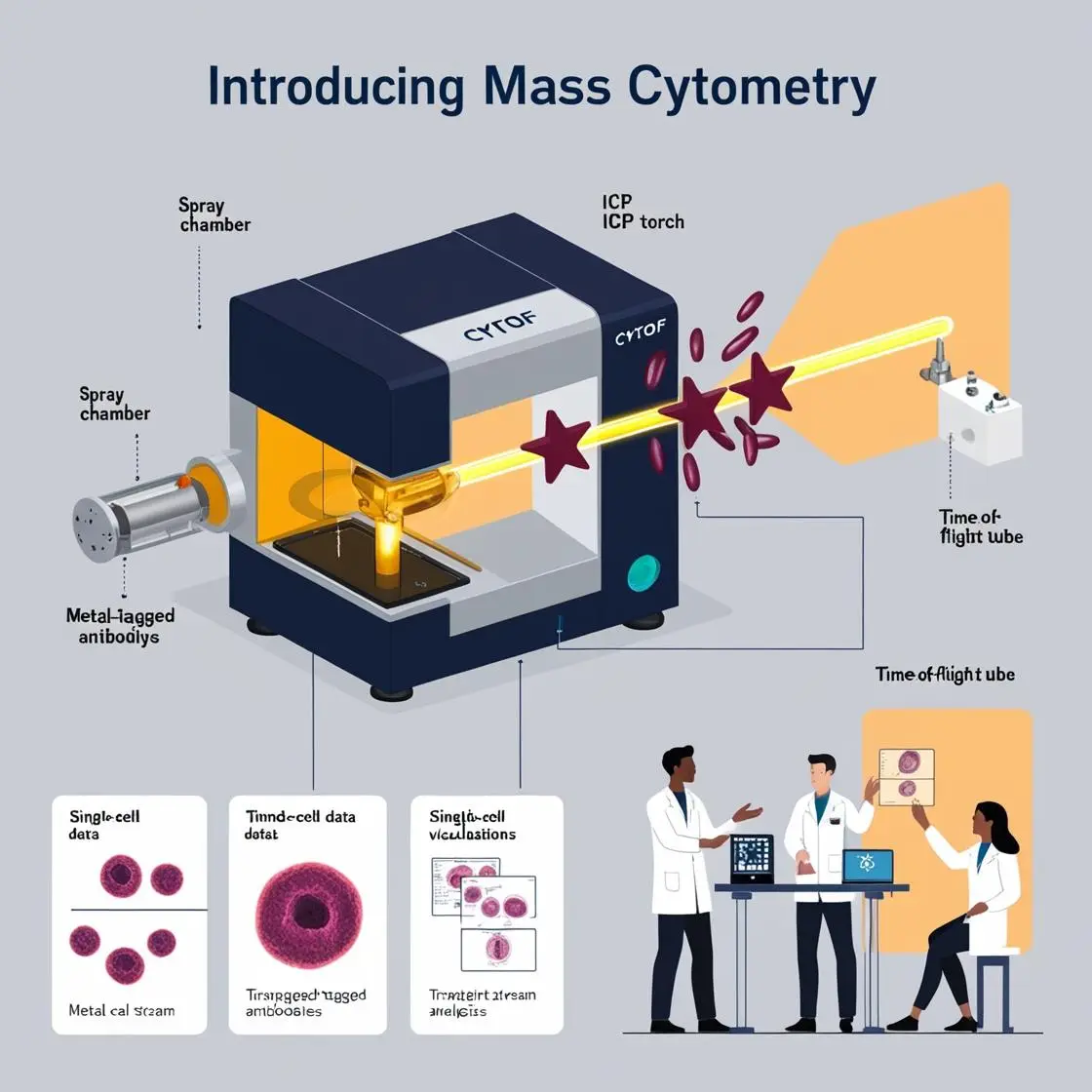
The convergence of artificial intelligence (AI) and mass cytometry has ushered in a new era of data analysis and interpretation in biomedical research and clinical practice. This powerful combination is revolutionizing our approach to understanding complex cellular systems and tailoring treatments for individual patients.
Deep Learning for Automated Cell Classification
One of the most impactful applications of AI in mass cytometry is automated cell classification. Traditional manual gating of cytometry data is time-consuming and subject to human bias. Deep learning algorithms have shown remarkable accuracy in identifying and classifying cell populations from high-dimensional cytometry data.
A landmark study by Li et al. (2019) published in Nature Methods, “Deep Cytometry: Deep learning with real-time inference in cell sorting and flow cytometry,” demonstrated how convolutional neural networks could be used for real-time cell classification in flow cytometry. While this study focused on flow cytometry, the principles have been successfully applied to mass cytometry data.
This paper introduces “deep cytometry,” a novel approach to cell analysis and sorting that combines label-free imaging with deep learning. Unlike traditional mass cytometry or flow cytometry, this method doesn’t require antibodies or any labeling. Instead, it uses time-stretch quantitative phase imaging (TS-QPI) to capture detailed information about cells’ physical properties as they flow through a microfluidic channel. A specialized laser and optical setup generate raw waveform data representing how light interacts with each cell. This data is then directly processed by a deep convolutional neural network, which can classify cells in real-time without the need for manual feature extraction or image conversion. The system achieved over 95% accuracy in distinguishing between cancer cells and white blood cells, and can make classification decisions within milliseconds, enabling real-time cell sorting. While it doesn’t provide the protein-specific information of mass cytometry or the dimensionality reduction of techniques like UMAP, this approach offers a complementary tool for rapid, label-free cell analysis based on physical and morphological properties.
Restricted content
You must be logged in and have a valid subscription to see this content. Please visit our subscription page for more info. If you are already a VIP member, be sure you are logged in with the same email address you made your purchase.