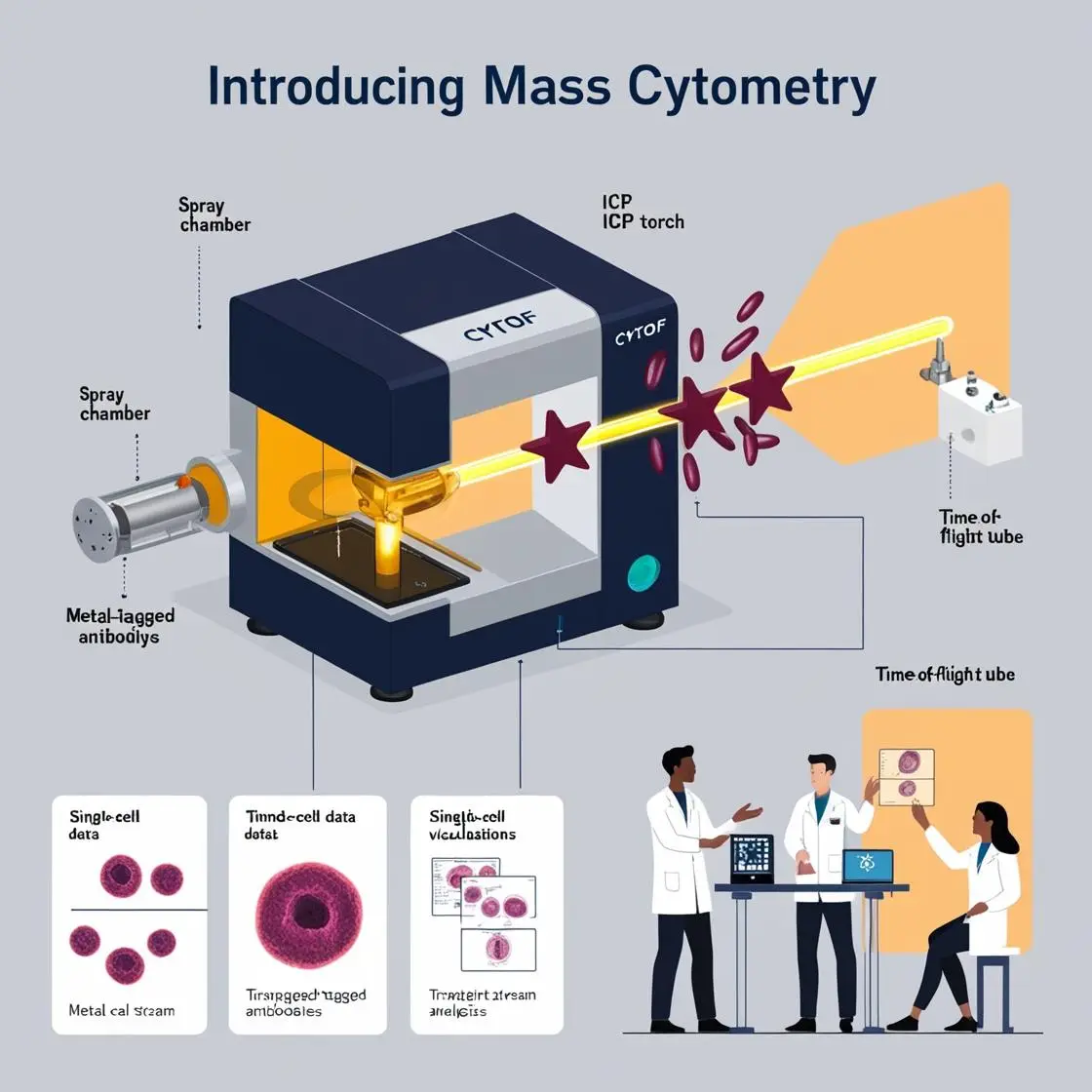
As we delve deeper into the vast oceans of data generated by mass cytometry, machine learning emerges as our trusty vessel, helping us navigate these complex waters and extract meaningful insights. Let’s embark on an exploration of how machine learning is revolutionizing mass cytometry data analysis.
Supervised and Unsupervised Learning Approaches
Machine learning in cytometry broadly falls into two categories: supervised and unsupervised learning.
Supervised Learning: In supervised learning, we train models on labeled data to predict outcomes or classify new, unseen data.
- Random Forests:
- Example: Aghaeepour et al. (2017) used random forests for automated cell population identification in their paper “An immune clock of human pregnancy” (Science Immunology, 2(15), eaan2946).
- Advantages: Random forests are robust to outliers and handle high-dimensional data well. They can capture complex, non-linear relationships in the data and provide measures of feature importance.
- Drawbacks: They can be computationally intensive for very large datasets and may overfit if not properly tuned. The model’s decision-making process can also be less interpretable compared to simpler methods.
- Support Vector Machines (SVM):
- Example: Greenplate et al. (2019) used SVM, among other machine learning methods, to analyze mass cytometry data and predict immunotherapy response in cancer patients in their paper “Computational immune monitoring reveals abnormal double-negative T cells present across human tumor types” (Cancer Immunology Research, 7(1), 86-99).
- Advantages: SVMs are effective in high-dimensional spaces, versatile through the use of different kernel functions, and work well when there’s a clear margin of separation between classes.
- Drawbacks: They can be sensitive to feature scaling, may perform poorly on highly imbalanced datasets, and can be computationally intensive for large-scale problems. Additionally, the choice of kernel and parameter tuning can significantly affect performance.
Unsupervised Learning: Unsupervised learning finds patterns in data without predefined labels.
- Principal Component Analysis (PCA):
- While not strictly machine learning, PCA is crucial for dimensionality reduction.
- Advantages: Simple, fast, preserves global structure.
- Drawbacks: Assumes linear relationships, can miss important non-linear patterns.
- t-SNE and UMAP:
- These are powerful visualization tools that have become staples in cytometry analysis.
- Advantages: Reveal local structure in high-dimensional data.
- Drawbacks: Can be computationally intensive, results can vary with parameter choices.
- Clustering Algorithms (e.g., FlowSOM, PhenoGraph):
- These help identify distinct cell populations.
- Advantages: Can reveal novel cell subsets, handle high-dimensional data.
- Drawbacks: Results can be sensitive to parameter choices, challenging to interpret biologically.

Deep Learning for Cytometry Data Analysis
Deep learning, a subset of machine learning based on artificial neural networks, has shown tremendous promise in cytometry data analysis.
Commercial Software Implementations: Several commercial platforms have incorporated deep learning and other machine learning tools for cytometry analysis:
- FlowJo (BD Biosciences): Offers plugins for automated population identification using FlowSOM and UMAP.
- Cytobank: Provides cloud-based analysis tools including CITRUS for automated cell population discovery.
- Astrolabe Diagnostics: Uses machine learning for automated cell population identification and analysis.
Challenges and Future Directions
While machine learning offers powerful tools for mass cytometry data analysis, several challenges remain:
- Interpretability: Many advanced models, especially deep learning models, act as “black boxes.” Improving model interpretability is crucial for biological insights.
- Data Integration: Combining mass cytometry data with other data types (e.g., genomics, clinical data) remains challenging but potentially very rewarding.
- Standardization: Developing standardized workflows and benchmarks for machine learning in cytometry is essential for reproducibility.
- Handling Batch Effects: Machine learning models need to be robust to technical variations between experiments.
- Scalability: As dataset sizes grow, developing scalable algorithms becomes increasingly important.
The future of machine learning in mass cytometry is bright, with emerging trends including:
- Transfer Learning: Using pre-trained models to improve performance on smaller datasets.
- Federated Learning: Allowing collaborative model training without sharing raw data, crucial for sensitive clinical data.
- Explainable AI: Developing models that not only make accurate predictions but also provide insights into the biological mechanisms behind those predictions.
As we navigate this exciting frontier, it’s crucial to remember that machine learning is a tool, not a magic wand. Its power lies in augmenting, not replacing, human expertise. By combining the pattern-recognition capabilities of machine learning with the domain knowledge of biologists and clinicians, we stand poised to unlock new insights from the rich data provided by mass cytometry.
In this dance between biology and algorithms, we’re not just analyzing data – we’re decoding the complex language of cells, one dataset at a time. The journey of discovery continues, with machine learning as our trusted companion in exploring the vast cellular universe revealed by mass cytometry.
In the great debate of scientific method, I found myself as the unsupervised clustering enthusiast, the data explorer without a map. While some scientists clung to their research questions like life rafts, I was busy tossing those rafts overboard and diving headfirst into the sea of unbiased discovery. For me, true science was about letting the data speak for itself, free from the shackles of our preconceived notions: so I was ready to fight any scientist asking me "But what is your research question?". CyTOF became my trusty submarine in this vast ocean of cellular data. With unsupervised clustering as my periscope, I was ready to spot patterns that no hypothesis-driven research would ever dream of. Some called it madness. I called it love at first cluster. Because in the end, isn't the most exciting question in science simply, "I wonder what we'll find?" And with CyTOF and unsupervised clustering, the answer was always, "Something unexpected!"
Guillaume Beyrend