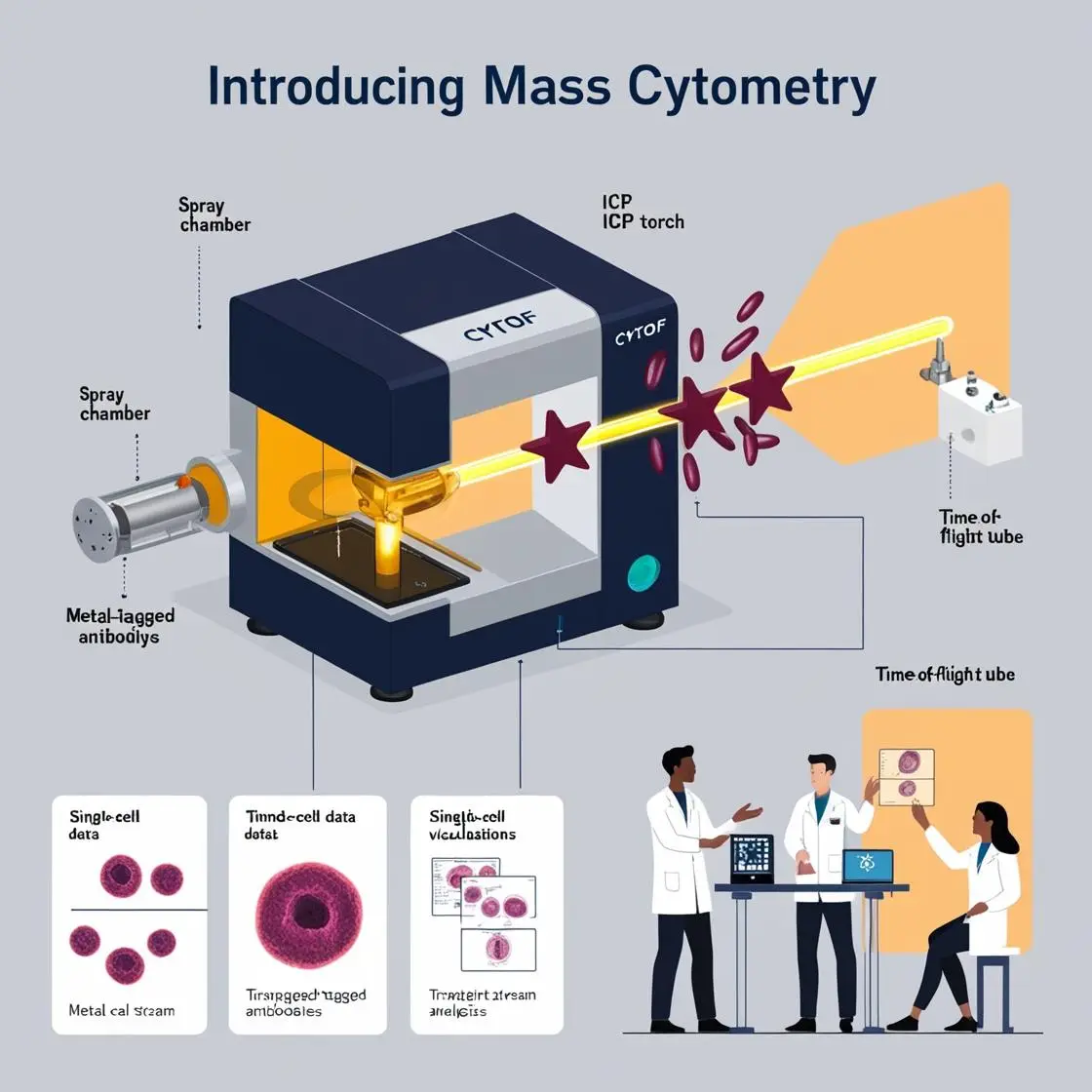
In the ever-evolving landscape of single-cell analysis, Imaging Mass Cytometry (IMC) stands as a revolutionary technique that bridges the gap between high-dimensional cytometry and histology. Let’s dive into this fascinating world where metal-tagged antibodies meet laser ablation to paint intricate cellular landscapes.
Principles of Imaging Mass Cytometry
Imaging Mass Cytometry, first introduced by Giesen et al. in 2014, Nature Methods, combines the high-dimensional capabilities of mass cytometry with spatial resolution at the tissue level. The basic principle is elegantly simple, yet technologically complex:
- Tissue Preparation: Formalin-fixed, paraffin-embedded (FFPE) or fresh-frozen tissue sections are mounted on slides and stained with metal-tagged antibodies, much like in traditional mass cytometry.
- Laser Ablation: A high-precision laser beam systematically moves across the tissue, ablating it pixel by pixel. Each laser pulse vaporizes a tiny spot of tissue, typically 1 μm in diameter.
- Mass Analysis: The ablated material is carried into a mass cytometer, where the metal tags are quantified, providing information about protein expression at each ablated spot.
- Image Reconstruction: The data from each ablated spot is compiled to reconstruct a high-dimensional image of the tissue, with each pixel containing information about multiple proteins.
A recent advancement in this field comes from the work of Ptacek et al. (2020) in “Nature Communications”. They introduced a method called MIBI-TOF (Multiplexed Ion Beam Imaging by Time of Flight), which uses an ion beam instead of a laser for ablation, achieving even higher spatial resolution.
Resolution and Capabilities
The resolution of IMC has seen significant improvements since its inception. The original system described by Giesen et al. (2014) achieved a resolution of 1 μm. However, recent advancements have pushed this further:
- The Hyperion Imaging System, a commercial IMC platform, offers a resolution of 1 μm for larger areas and can achieve subcellular resolution of 280 nm for smaller regions of interest.
- The MIBI-TOF system introduced by Ptacek et al. (2020) can achieve resolutions as high as 260 nm, rivaling super-resolution light microscopy.
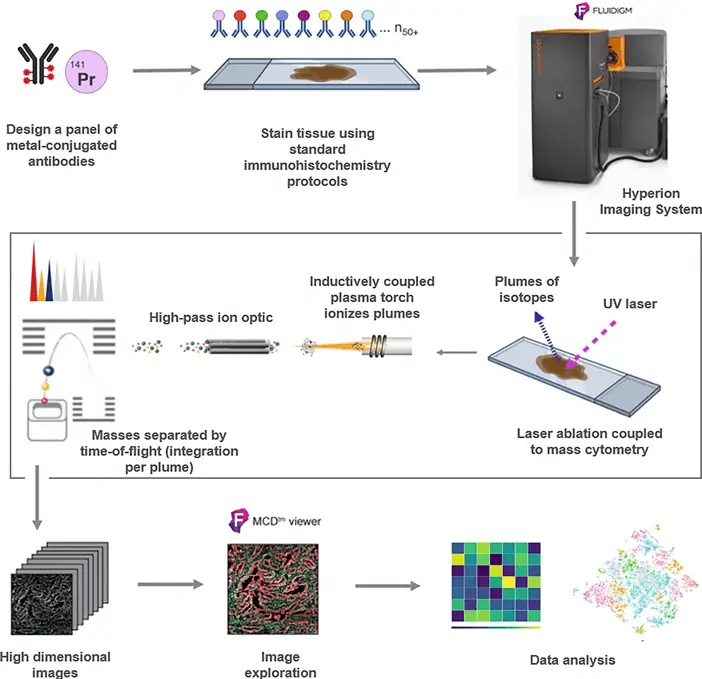
In terms of multiplexing, IMC truly shines. While traditional immunohistochemistry is typically limited to 3-4 markers per section, IMC can simultaneously visualize 40 or more proteins in a single tissue section. This capability allows for unprecedented characterization of cellular phenotypes and their spatial relationships within tissues.
There is even now a “Three-dimensional imaging mass cytometry for highly multiplexed molecular and cellular mapping of tissues and the tumor microenvironment”, Technical Report, 2021.
Comparison with Traditional Immunohistochemistry
IMC offers several advantages over traditional immunohistochemistry (IHC):
- Higher Multiplexing: As mentioned, IMC can measure 40+ proteins simultaneously, compared to 3-4 in IHC.
- Quantitative Measurements: Unlike the semi-quantitative nature of IHC, IMC provides truly quantitative data for each marker.
- No Autofluorescence: IMC is not affected by tissue autofluorescence, a common problem in fluorescence-based techniques.
- Preserved Morphology: The use of metal tags instead of bulky fluorophores helps preserve fine subcellular structures.
However, IMC also has some limitations:
- Lower Throughput: IMC is significantly slower than traditional IHC or even multiplex immunofluorescence.
- Destructive: The tissue is ablated during analysis, preventing reanalysis of the same section.
Applications in Tissue Analysis
The applications of IMC are vast and growing. Here are a few exciting areas:
- In cancer research, IMC has been particularly impactful. In their paper, cite over 1600 times, “Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry”, Giesen et al. gained spatial information by coupling immunohistochemical and immunocytochemical methods with high-resolution laser ablation to CyTOF mass cytometry. This groundbreaking study demonstrated the capability of IMC to simultaneously image 32 proteins and protein modifications at subcellular resolution on breast cancer tissues. It laid the foundation for high-dimensional tissue imaging in cancer research.
- In their paper, issued by the Nolan group with Schürch et al. in Cell, 2020, researchers identified distinct cellular neighborhoods within tumors and found that the organization of these neighborhoods was associated with patient survival. “Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front” showed that tumors with well-organized immune cell clusters, particularly those rich in PD-1+ T cells adjacent to PD-L1+ macrophages and tumor cells, were associated with improved patient outcomes. This work highlighted the importance of spatial organization in anti-tumor immunity and provided insights that could inform immunotherapy strategies.
- More recently, in April 2024, researchers have developed a 42 marker panel for in-depth study of cancer associated fibroblast niches in breast cancer using imaging mass cytometry.
Practical Considerations
One of the unique aspects of IMC is its operational characteristics. Unlike flow cytometry, which requires constant supervision, IMC systems can run autonomously for extended periods. This is both a blessing and a challenge:
- Advantage: Once set up, an IMC run can proceed unattended, even overnight. This allows for efficient use of instrument time and researcher effort.
- Challenge: A single run can take many hours to complete. For example, imaging a 1 mm² area at 1 μm resolution can take 2-3 hours.
Interestingly, IMC systems don’t face the clogging issues common in suspension mass or flow cytometry. The laser ablation process ensures a steady stream of single-cell data without the risk of cell clumps or debris causing blockages.
As we look to the future, IMC stands poised to revolutionize our understanding of tissue biology. By providing high-dimensional, spatially resolved data at subcellular resolution, it offers a window into the complex cellular choreography that underlies both health and disease. While challenges remain in data analysis and throughput, ongoing advancements promise to further cement IMC’s place as a cornerstone technology in the pathologist’s and researcher’s toolkit.
From unraveling the mysteries of the tumor microenvironment to mapping the intricate processes of development, IMC is helping us paint the most detailed pictures yet of life’s cellular landscapes. As we continue to push the boundaries of resolution, multiplexing, and analysis, who knows what new biological vistas will open up before us?
In my department where I worked as a PhD student, imaging mass cytometry burst onto the scene like a supernova, blurring the line between scientist and artist. Suddenly, we were painting with proteins, sculpting with antibodies. PhD students from our group envisioned thesis covers to make National Geographic jealous. "Forty markers at once!" we'd exclaim, ignoring that even Rembrandt (born actually in Leiden, the town where I did my PhD) would struggle to represent that many variables meaningfully. Some groups made claims that would make Merlin raise an eyebrow. "We can visualize every protein in every cell simultaneously!" they'd declare, glossing over the complexity of such a feat. The results? Often a glorious collision of disco and biology - dazzling, perplexing, and not always interpretable. But oh, the excitement! The spectacle! In the end, we learned: in science, as in art, sometimes less is more. Unless you're aiming for the "Most Kaleidoscopic Thesis" award - then, paint on, you cellular Picasso!
Guillaume Beyrend